Meet ImmuneCyte
ImmuneCyte Inc. is a global, clinical-stage biopharmaceutical company engaged in the research and development of innovative cell and gene therapeutics, including chimeric antigen receptor (CAR)-T cell, CAR-NK cell, and mononuclear cell therapies, for the treatment of cancer, vascular, orthopedic and aging-related diseases. We are a privately held company with headquarter in Irvine, California and access to 30,000 sq. ft. of GMP-qualified laboratory and office facilities in Tianjin, China. The location of our operations provides us with many potential strategic advantages, including proximity to world’s largest pharmaceutical market and access to largest number of patients.
We build our immuno-oncology pipeline based on our proprietary CARTXpress™ platform, licensed from one of our parent companies, ThermoGenesis Holdings Inc. The technology platforms were developed in the U.S. by a dedicated professional team of biologists, engineers and other clinical professionals. We leverage this high-efficient and versatile cellular processing and manufacturing platform to develop a robust pipeline of cell and gene therapy products for the treatment of cancers and rare genetic disorders. Currently, we are in clinical stage of developing several unique chimeric antigen receptor (CAR) T cell and natural killer cell-based therapeutics for advanced hematopoietic cancers and solid tumors. We have also used this platform to develop advanced cell-based gene therapies for the treatment of severe cases of b-thalassemia, a devastating blood genetic disorder that affects more than 60,000 infants each year.
ImmunoOncology
CAR-T & NK Therapy
Tumor Infiltrating Lymphocyte
Dendritic Cell Therapy
Key Technology Platforms
- CARTXpress™ is our proprietary manufacturing platform for cell-based immunotherapies, which could potentially reduce the manufacturing cost by two thirds, making our immunotherapies highly competitive in cell and gene therapy field
Lead Product Candidate
- BCM-5011 is an autologous hCD-19 CAR-T cell therapy for B cell malignancy
- BCM-5021 is an autologous TIL therapy for solid tumor cancer
- BCM-5031 is an autologous DC/CIK therapy for solid tumor cancer
- BCM-6100 is a newly developed CAR-NK2GD platform for various solid tumor cancer
Highlights
- Our proprietary manufacturing platform CARTXpress allows we build a Best-in-class strategy for immno-oncology pipeline.
- BCM-5011 estimated to cost ONLY 1/3 the price of the two approved CAR-T drugs, making it highly competitive.
- Using proprietary X-BACS separation technology, BCM-5021 is able to effectively select and expand the tumor infiltrating lymphocytes from solid tumor, and use it as effective therapy to fight solid tumor.
- BCM-5031 is used in combination with surgical procedures to fight for micro-metastasis in solid tumor cancer.
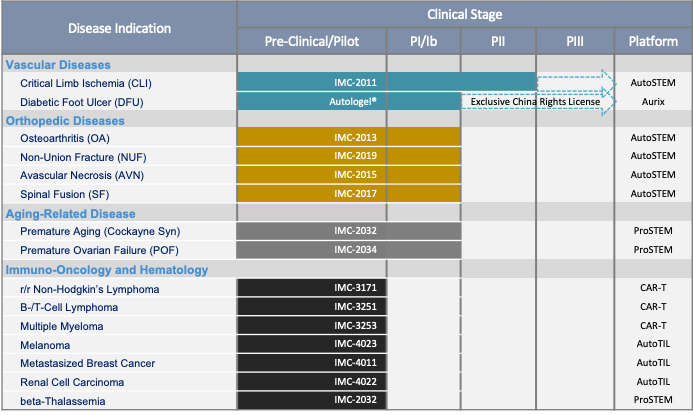
Pipeline
Leadership
Executive Team

Chris Xu
PhD, MBA | Chief Executive Officer

Michelle Zhu
President

Paul V, Holland
MD | Chief Medical Officer

Joseph Shen
MBA | Chief Operating and Marketing Officer

Chris Xu
PhD, MBA | Chief Executive Officer

Michelle Zhu
President

Paul V, Holland
MD | Chief Medical Officer

Joseph Shen
MBA | Chief Operating and Marketing Officer

Jody Patterson
Head of Quality

Jody Patterson
Head of Quality
Board of Directors

Dr. Chris Xu
PhD, MBA | Chairman

Han Ying
PHD, Director

Michelle Zhu
Director
News
COVID-19 News
ImmunoOncology News
No feed items found.
ImmuneCyte News

Leave A Message
©2020 All rights reserved. ImmuneCyte, Inc.
A Healthbanks Biotech Company


